Whitepaper
Whitepaper Download
Unique Device Identifier
The unique device identifier (UDI) is a unique number on medical devices that identifies a device at the point of distribution and at the point of use. The UDI consists of the following:
Global Trade Item Number (GTIN) + Application Identifier (AI) = UDI
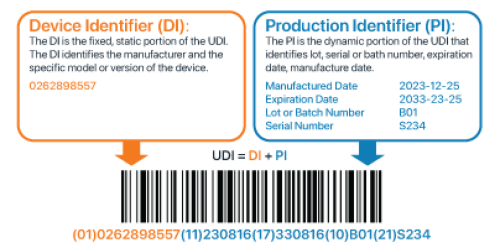
Medical devices and in vitro diagnostic (IVD) devices will require a UDI, unless otherwise exempt by the TGA.
UDIs will apply to the following medical devices and IVD devices:
- Class III
- Class IIb
- Class IIa
- Class Is – supplied sterile
- Class 4
- Class 3
- Class 2
- Class 1 categorised as:
- Instrument/analyser (GMDN Collective Term 943)
- Software (GMDN Collective Term 944)
Some medical devices already have a UDI on the device, the labelling or packaging due to UDI being implemented globally.
Australian Unique Device Identifier Database (AusUDID)
The TGA has established the Australian UDI Database (AusUDID) as a repository of medical device UDI information for devices supplied in Australia. This data in will link to relevant medical device in the Australian Register of Therapeutic Goods (ARTG).
AusUDID will enable you to search, view and download UDI information. The information stored in AusUDID will contain:
- DI
- Brand name
- Manufacturer name
- Sponsor name
- GMDN
- ARTG ID
- Patient information leaflet
Further guidance on using the AusUDID will be published on the UDI Hub in 2024. Click link here to UDI Hub. https://www.tga.gov.au/how-we-regulate/manufacturing/manufacture-medical-device/unique-device-identification-udi-hub
UDI Compliance Timeframe
Mandatory compliance will be progressively phased by the TGA based on device classification. The TGA will begin with high-risk and implantable medical devices, followed by lower risk class devices.
Mandatory compliance will commence at a minimum of 12 months from the date the regulations take effect. The dates for compliance are yet to be announced. Please refer to the TGA website for updates.